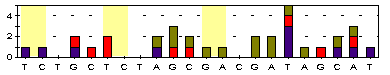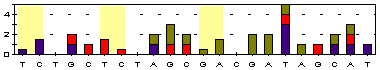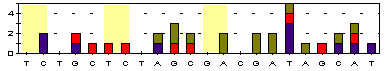_
|
|
||||||||||||||||||||||||||||||
|
Pyrosequencing
protocol for detection of FV, FII and MTHFR
|
|||||||||||||||||||||||||||||||
|
The sequences of PCR and sequencing primers (Thermo Electron) used for each assay are shown below. Amplicons were generated in a 50µl reaction volume with 15pmol of forward and reverse PCR primers, 0.2mM dNTPs (Promega), 1.5mM MgCl2, 1X Buffer II (Applied Biosystems), 1U AmpliTaq Gold (Applied Biosystems) using 10ng genomic DNA. PCR conditions were 94°C for 7 min; 50 cycles with denaturation at 94°C for 30s, annealing at 60°C for 30s and elongation at 72°C for 30s; 1 cycle at 72°C for 7 min; and a final hold at 4°C. Thermocycling was performed using a PTC-0225 DNA Engine Tetrad (MJ Research). Single-stranded biotinylated
PCR products were prepared for sequencing using the Pyrosequencing™ Vacuum Prep Tool. 3µl Streptavidin
Sepharose™ HP (Amersham) was added to 37µl Binding
buffer (10 mM Tris-HCl pH 7.6, 2M NaCl, 1 mM EDTA, 0.1% Tween 20)
and mixed with 20µl PCR product and 20µl high purity
water for 10 min at room temperature using a Variomag Monoshaker
(Camlab). The beads containing the immobilised templates were captured
onto the filter probes after applying the vacuum and then washed
with 70% ethanol for 5 sec, denaturation solution (0.2M NaOH) for
5 sec and washing buffer (10 mM Tris-Acetate pH 7.6) for 5 sec.
The vacuum was then released and the beads released into a PSQ
96 Plate Low containing 45µl annealing buffer (20 mM Tris-Acetate,
2 mM MgAc2 pH 7.6), 0.3µM sequencing primer.
T/T T/T G/G T/C T/C G/A C/C C/C A/A
|
|||||||||||||||||||||||||||||||
Last Updated: 7 August, 2008 by G. Watkins |
|||||||||||||||||||||||||||||||